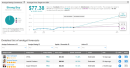
AstraZeneca has secured $486m from the US Government for the development and supply of 100,000 doses of its potential AZD7442 antibody cocktail for the treatment of Covid-19.Under the current agreement, AstraZeneca (AZN) plans to supply up to 100,000 doses towards the end of 2020. In addition, the US government has an option to buy up to another 1 million doses in 2021.AZD7442, which is a combination of 2 long-acting antibodies (LAABs), will be evaluated in 2 Phase 3 clinical trials. The two AZD7442 trials, which will enroll over 6,000 adults testing the antibody cocktail for the prevention of Covid-19 at sites in and outside the US, are due to begin in the next few weeks. One trial will evaluate the safety and efficacy of AZD7442 to prevent infection for up to 12 months, in approximately 5,000 participants. The second trial will evaluate post-exposure prophylaxis and pre-emptive treatment in approximately 1,100 participants.Additional trials are planned with the enrollment of about 4,000 adults for the treatment of SARS-CoV-2 infections.“This agreement with the US government will help accelerate the development of our long-acting antibody combination which has the potential to provide immediate and long-lasting effect in both preventing and treating Covid-19 infections,” AstraZeneca CEO Pascal Soriot said. “We will be evaluating the LAAB combination in different settings from prophylaxis, to outpatient treatment to hospitalisation, with a focus on helping the most vulnerable people.”The LAABs have been engineered with AstraZeneca’s proprietary half-life extension technology to increase the durability of the therapy for 6 to 12 months following a single administration. The combination of two LAABs is also designed to reduce the risk of resistance developed by the SARS-CoV-2 virus, the company said.LAABs mimic natural antibodies and according to AstraZeneca have the potential to treat and prevent disease progression in patients already infected with the virus, as well as to be given as a preventative intervention prior to exposure to the virus. As such, a LAAB combination could be complementary to vaccines as a prophylactic treatment, for example for people for whom a vaccine may not be appropriate or to provide added protection for high-risk populations. It could also be used to treat people who have been infected, the company said.AZN shares have gained almost 10% this year as the drugmaker joined the list of companies engaged in the development of a potential coronavirus vaccine. The Phase 2/3 study of the AstraZeneca/Oxford vaccine candidate was last month put on hold after an adverse event in a single participant from the UK. The trial has been restarted in the UK but is still on hold in the US. (See AstraZeneca stock analysis on TipRanks)SVB Leerink analyst Andrew Berens recently reiterated a Buy rating on the stock with a $65 price target suggesting that the adverse event could have a broader impact and could cause near-term volatility in AstraZeneca’s shares as well as in the stock of other companies with Covid vaccine programs until the exact nature of the event is clear.As a result, Berens cautions that the overall speed of many of the programs could be affected as the investigation progresses and sponsors become more vigilant, as well as public sentiment regarding the safety of the vaccines once approved.Overall, AZN scores a Strong Buy consensus from the analyst community with 4 unanimous Buy ratings. Looking ahead, the $77.38 average analyst price target puts the upside potential at a promising 41% in the coming 12 months.Related News: CareDx Soars 15% As 3Q Revenue Outlook Surpasses Estimates BioNTech, Rentschler Partner For Covid-19 Vaccine Manufacturing Gilead Inks EU Supply Deal For 500,000 Remdesivir Doses More recent articles from Smarter Analyst: * Apple Allowed To Block Fortnite From App Store Amid Dispute * Microsoft or Apple: Which Tech Giant Is A Better Pick? * Air Canada Chops Transit Takeover Price By 74% Due To Covid-19 Crisis * Ameren Ramps Up Quarterly Dividend By 4%,
,
