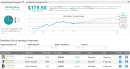
Eli Lilly & Co. announced a definitive agreement to snap up privately-held biotechnology company Disarm Therapeutics for $135 million, in a bid to a develop a new class of disease-modifying therapeutics for patients with axonal degeneration.Under the terms of the agreement, Disarm shareholders will be eligible for up to $1.225 billion in future payments for potential development, regulatory and commercial milestones should Lilly (LLY) successfully develop and commercialize new medicines resulting from the acquisition. Axonal degeneration is a common, yet unaddressed, pathology in a broad range of neurological diseases and is known to cause severe sensory, motor, and cognitive symptoms.Disarm has discovered novel, potent SARM1 inhibitors and is advancing them in preclinical development, with the goal of delivering treatments to patients with peripheral neuropathy and other neurological diseases such as amyotrophic lateral sclerosis (ALS) and multiple sclerosis. The biotech company found that SARM1 protein is a central driver of axonal degeneration. As such, SARM1 inhibitors are designed to directly prevent the loss of axons.”Lilly continues to seek medicines to treat the debilitating pain and loss of function associated with nerve damage,” said Lilly’s Mark Mintun. “The scientific team at Disarm discovered an important and highly promising approach to combat axonal degeneration. We will move quickly to develop their SARM1 inhibitors into potential medicines for peripheral neuropathy and neurological diseases, such as ALS and multiple sclerosis.”Lilly said that the transaction won’t have any impact on its 2020 non-GAAP earnings per share guidance.Shares in LLY are up more than 11% year-to-date, but have dropped almost 7% over the past 5 days as the Phase 3 trial of its leading monoclonal antibody coronavirus treatment has been paused by the US Food and Drug Administration (FDA) over potential safety concerns.Earlier this month, Lilly announced that it had applied for emergency use authorization (EUA) for one of its Covid-19 antibody candidates, LY-CoV555, as a monotherapy for higher risk patients with mild-to-moderate symptoms. The company also presented new interim trial data for the combination therapy of LY-CoV555 and another antibody, LY-CoV016.While Mizuho analyst Vamil Divan viewed the new data as “promising,” the analyst believes the “clinical meaningfulness of some datapoints remains up for debate.” Divan reiterated a Hold rating on the stock with a $164 price target (12% upside potential).“We wonder where the combination would fall in the Covid treatment algorithm, how/where patients would receive it, and how Lilly may price the therapy, but overall find today’s data release encouraging,” Divan wrote in a note to investors on Oct. 7. (See Eli Lilly’s stock analysis on TipRanks).Overall, the stock scores a bullish Strong Buy Street analyst consensus. That’s with a $175.50 average analyst price target, indicating 20% upside potential lies ahead.Related News: Eli Lilly Pauses Late-Stage Covid-19 Antibody Trial Over Safety Concerns PerkinElmer Raises 3Q Sales Outlook Fueled By COVID-19 Testing Demand J&J Halts Covid-19 Vaccine Trial Due To ‘Unexplained Illness’ More recent articles from Smarter Analyst: * Loop Drops 6% On SEC Subpoena; Roth Sees 104% Upside * Macy’s Or TJX: Which Retail Stock Is The Street’s Better Pick? * Boeing On Cusp Of EU Approval For Grounded 737-MAX Jet – Report * Pfizer Targets FDA Approval For Covid-19 Vaccine In November; Shares Rise,
,
