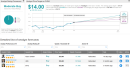
Shares in Fluidigm spiked 16% in Friday’s extended market session after the company’s Covid-19 saliva test was selected by the Trump Administration for use in a US federal program at testing sites in Texas.Waco, Texas, is the first community to offer Covid-19 saliva testing through the US federal program starting October 16. The Fluidigm (FLDM) saliva test will be offered at 3 sites through mid-November. Fluidigm’s test that uses samples of saliva collected by spitting into a sterile container, detects SARS-CoV-2, the virus that causes Covid-19, on a molecular level using a microfluidics platform.The US federal program provides Covid-19 testing sites for a limited period in areas where there has been a recent and significant increase in the number of new virus cases and hospitalizations. The program is administered by the U.S. Department of Health and Human Services (HHS) in partnership with local communities and private companies. HHS has shipped 10,000 Fluidigm saliva tests to select Waco, Texas sites.The Fluidigm integrated microfluidics platform used in the saliva test can generate as many as 6,000 test results per day. With many existing Fluidigm instruments in clinical and research labs throughout the U.S., the company is now expanding its microfluidics manufacturing capacity and developing a novel barcoding chemistry to further boost testing.As it is saliva-based, the Advanta Dx SARS-CoV-2 RT-PCR test does not require collection via invasive nasopharyngeal swab. The accuracy of the test is comparable to other molecular-level tests of nasal swab samples, according to results from a clinical study. Back in August Fluidigm received Emergency Use Authorization (EUA) from the U.S. Food and Drug Administration (FDA) for the saliva test.Shares in FLDM, which have spiked over 94% year-to-date, are down 13% over the past 5 days. The stock scores a cautiously optimistic Moderate Buy Street consensus based on 2 recent Buy ratings. Meanwhile the $14 average analyst price target indicates a promising 107% further upside potential from current levels.Piper Sandler’s Steven Mah at the end of last month reiterated a Buy rating on the stock with a $12 price target (78% upside potential), noting that FLDM “has opportunistically shifted to Covid-19 testing”.Mah believes that CLIA lab testing and rapid point-of-care (POC) testing can “co-exist without cannibalizing each other”. The analyst added that he would be a buyer of Fluidigm given the recent pullback. (See FLDM stock analysis on TipRanks)Related News: Eli Lilly Pauses Late-Stage Covid-19 Antibody Trial Over Safety Concerns PerkinElmer Raises 3Q Sales Outlook Fueled By COVID-19 Testing Demand J&J Halts Covid-19 Vaccine Trial Due To ‘Unexplained Illness’ More recent articles from Smarter Analyst: * First Citizens, CIT To Merge Into $100B US Regional Lender; Shares Spike * Loop Drops 6% On SEC Subpoena; Roth Sees 104% Upside * Macy’s Or TJX: Which Retail Stock Is The Street’s Better Pick? * Eli Lilly To Snap Up Disarm In $135M Deal; Street Stays Bullish,
,
